The Nitrification Process
Nitrogen loss from NO3- leaching can be anywhere from 10 – 40% and can lead to economic opportunity loss to the grower and environmental consequences. With moderate temperatures and soil moisture, nitrification can occur on moist soils within a few weeks. But what is nitrification?
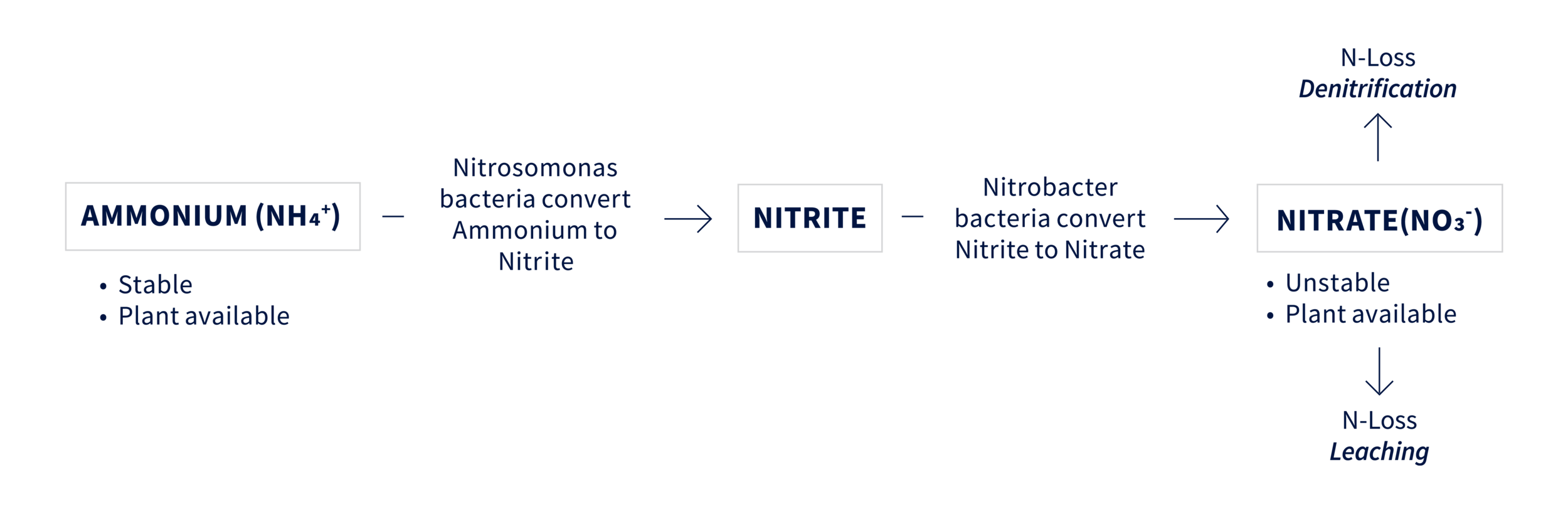
Firstly, when applied to the soil, urea (CO(NH2)2) and ammonia (NH3 gas) based fertilizers convert to ammonium (NH4+) in a mineralization process called ammonification. Ammonium nitrogen is plant available and stable until nitrification starts the conversion process.
Nitrification is a natural two-step process of ammonium (NH4+) nitrogen converting to nitrate (NO3-) in the soil. Ammonium is first converted to nitrite (NO2-) by the Nitrosomonas bacteria and then to nitrate (NO3-) by the Nitrobacter bacteria.
Mineralization and nitrification are influenced by environmental factors that affect biological activity such as temperature, moisture, aeration and pH. Studies by the University of Nebraska show that nitrification occurs slowly at cold temperatures and increases as temperatures rise, with the biggest growth from 52°F - 75°F.
(Nebraska Extension EC 155, pg. 6)
There are ways to minimize the risk of leaching. The 4R Principles are effective guidelines for the grower to work with their crop consultant. Equally, the rate of nitrification can be reduced by keeping N in the ammonium form longer, allowing it to bind to the negatively charged soil. Nitrification inhibitors, such as DCD, slow the conversion of ammonium to nitrate by temporarily disrupting the activity of the Nitrosomonas bacteria. DCD is an AAPFCO defined nitrification inhibitor. It is non-corrosive and not a labeled pesticide.
Author: Ethan Enochs